OnderLaw is dedicated to helping women who have experienced pain, suffering, and medical bills related to use of transvaginal mesh, and to making sure that dangerous products like it are pulled from profit-driven use, regardless of the harm they may cause.

Thursday, May 23, 2019 –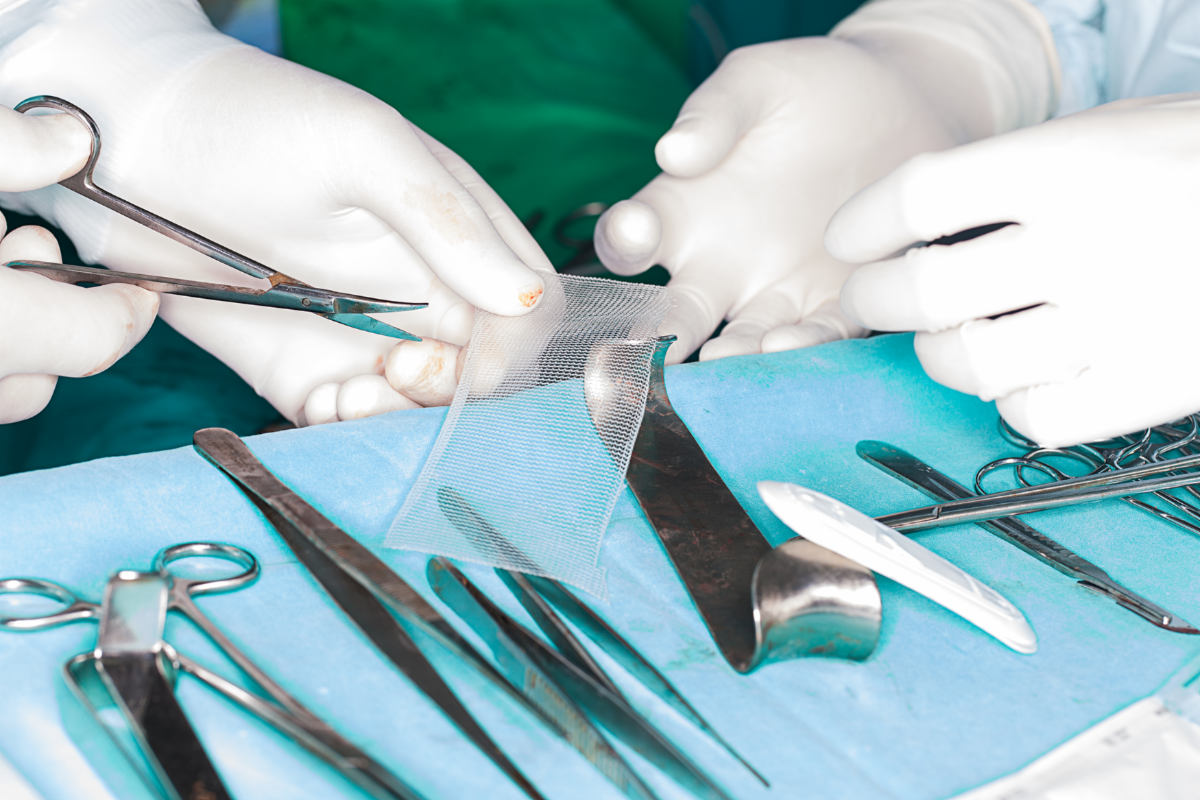
For years, transvaginal mesh was used despite warnings from the FDA that it could be dangerous, and even though there were other medical interventions available that could have reduced the risk of complications.
Here’s what you need to know.
Transvaginal mesh is a product that has been used to treat pelvic floor disorders, including pelvic organ prolapse and stress urinary incontinence. Though it was cleared for use by the FDA in 2002 as a Class II, or moderate risk treatment, it wasn’t long before women began experiencing serious problems with transvaginal mesh.
In 2008, the FDA began to raise a red flag, cautioning patients that, that year, they’d received reports that over 1,000 women had experienced pelvic pain, infection, bleeding, organ perforation, urinary problems, and vaginal scarring, among other complications.
Two years later, in 2011, the FDA raised a second warning, saying that these complications were “not rare.” They urged patients and doctors alike to “be aware of the risks” before using transvaginal mesh.
Most commonly, the mesh was eroding through patients’ vaginal walls. Women have faced serious problems as a result, and even after multiple surgeries, some women still struggle with medical issues.
Use Increased Despite Problems
Despite the warnings, use of transvaginal mesh actually increased. One 2015 study showed that, in New York alone, the number of cases in which transvaginal mesh was used increased by 44.7% from 2008, when the first warning was issued, to 2011.
The reason: transvaginal mesh is big business. According to Dr. Shlomo Raz, professor of urology and pelvic reconstruction at UCLA, 3 to 4 million women have had transvaginal mesh implanted. Complications have been reported in about 5% of those women.
As use increased, lawsuits began to roll in. By the middle of 2014, nearly 60,000 suits had been filed against Boston Scientific and Coloplast, two of the five manufacturers of transvaginal mesh.
FDA Steps In
In January 2016, the FDA reclassified transvaginal surgical mesh used for pelvic organ prolapse repair, forcing manufacturers to submit their devices for FDA scrutiny before they could be used. As a result, manufacturers quit marketing transvaginal mesh for use in what’s called “rectocele,” which is a type of prolapse repair.
Earlier this month, April 16, 2019, the FDA ordered manufacturers to stop selling and distributing transvaginal mesh for another type of repair, called “cystocele.” As a result of this order, there are no longer any transvaginal mesh products that can be marketed in the United States for transvaginal repair.
Fighting the Good Fight
This order only occurred because women were willing to stand up through the court system and demand change. There is still a fight left to battle, however. The manufacturers need a clear message that they cannot put lives at risk for the sake of profits.
Our hearts go out to women and their families who have been negatively affected by the use of transvaginal mesh. OnderLaw currently represents women from across the country who have suffered due to the prolonged use of transvaginal mesh, even after manufacturers had become aware that it was risky. It’s important to send a message that people must not be used as pawns in a high-profit game of risk.
If you have experienced symptoms of transvaginal mesh complications, including erosion of the mesh through the vagina, pelvic pain, chronic inflammation, serious infection, bleeding, painful sexual intercourse, organ perforation, pelvic pain or swelling, or abdominal or buttock pain, Contact us today . We have a long history of representing clients harmed by dangerous drugs and defective medical devices.
We welcome surgical mesh inquiries from law firms interested in sharing information or working as co-counsel.