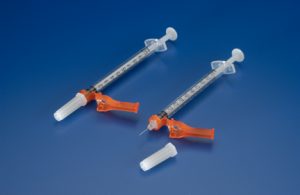
 Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringes, manufactured by Smiths Medical, have been recalled due to skewed line graduation markings that could result in dangerous insulin injections. This is a Class I recall, indicting it is the most urgent type of recall due to the potential for serious harm.
Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringes, manufactured by Smiths Medical, have been recalled due to skewed line graduation markings that could result in dangerous insulin injections. This is a Class I recall, indicting it is the most urgent type of recall due to the potential for serious harm.
According to a statement issued by the Minneapolis-based company, markings can be skewed approximately 20 degrees upward. Incorrect insulin administration is possible with these needles, which could result in hyperglycemia or hypoglycemia. Serious harm, including ketoacidosis, seizures, or death could result.
The Following Models and Lot Numbers are Affected:
|
Model |
Name |
Lot Number |
|---|---|---|
| 4428-1 | Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringe 28Gx1/2” 1CC | 4046543, 4062235 |
| 4429-1 | Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringe 29Gx1/2” 1CC | 4014096, 4031846, 4031845, 4040734, 4043536, 4046545, 4046546, 4062239, 4062240, 4062238 and 4062242 |
The FDA has not received reports of deaths or serious injuries from this issue. Smiths Medical has issued recall notices to retailers, home healthcare and medical providers that distribute affected needles, and they have been instructed to return any and all unused products.
Consumers should return the needles to retailers, home healthcare providers, or medical offices where they were obtained for replacements.
If you have been seriously injured by a dangerous medical product or drug, contact OnderLaw for a free, no-obligation consultation. Our personal injury lawyers hold corporations accountable when they put profits over the safety of people.