 What is Behind Exactech Lawsuits?
What is Behind Exactech Lawsuits?
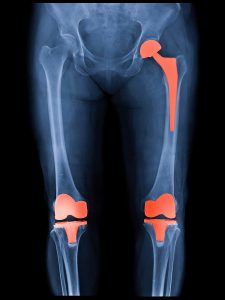
Hundreds of thousands of people who underwent hip and knee replacement surgeries are now eligible to file a lawsuit for their faulty implants. Exactech, headquartered in Gainesville, Florida, has removed all models of hip and knee replacements using their polyethylene liners from the market and issued letters to surgeons and physicians advising them to remove and replace them due to faulty liners.
Polyethylene liners act as shock absorbers in joint replacements. They replace the function of cartilage, and when they are faulty, as they are with Exactech inserts, they cause undue pain, swelling, difficulty walking, degradation of bone, implant fracture, and other serious implications.
Interestingly, the inserts themselves have not been the source of the problem. Prior to being used in patients, they have been stored in defective vacuum bags that allowed them to be exposed to oxygen. These defective bags did not contain a second layer of ethylene vinyl alcohol, which would have likely protected the hip and knee replacement parts from degenerating.
Patients who received implants using Exactech inserts may be eligible for compensation due to the unnecessary pain and suffering of having to go through corrective surgery. Exactech lawsuits are among the latest hip and knee lawsuits that are being filed to help these victims to seek settlement in court.
Exactech, Inc. makes polyethylene joint replacement liner components. These are used in hundreds of thousands of hip and knee implant systems that have been used since 2004.
Exactech Hip Replacement Recall Lawsuit Attorneys
On June 28, 2021, Exactech, Inc. issued “Urgent Medical Device Correction” letters to distributors, physicians, and anyone else who sells or uses hip implants using their components. According to the United States Food and Drug Administration (FDA) class 2 recall notice, Exactech has recalled numerous Connexion GXL acetabular polyethylene hip liners used in hip implant systems.
The reason for the recall, as stated on the FDA’s recall notice is because of a risk “of edge-loading and premature prosthesis wear . . . in a specific subset of patients with certain implant configurations and surgical implant positioning.” Exactech polyethelene inserts, also called Exactech Ultra-High Molecular Weight Polyethylene (UHMWPE), can fail, causing pain, wearing of the joint, bone loss, and failure and fracture of the implant.
As part of this recall, physicians are advised to remove the implants. For those who have these hip implants, revision surgery is painful and can result in lost wages and loss in quality of life. Those affected can file a hip replacement Exactech lawsuit to be compensated.
OnderLaw has been at the forefront of these types of lawsuits, called mass torts, in the United States. We have represented approximately 70,000 people just like you who have been victims of corporations that put profits over the lives of people.
If you have an Exactech hip replacement and believe it may be part of the recall, contact OnderLaw today!
Call 314-408-6424
from anywhere in the United States!
OnderLaw hip and knee replacement lawyers can help.
Our consultations are free.
You don’t pay a dime unless we win your case.
Exactech Knee Replacement Recall Lawsuit Lawyers
On February 7, 2022, Exactech issued a knee replacement recall notice warning of over 100,000 defective and dangerous knee inserts that had been used in patients since 2004. Exactech kept this recall relatively quiet until news got out in 2022. The recall, which began with about 60,000 inserts, has now been expanded to include Exactech Optetrak knee replacements sold since 2004, Logic knee implants sold since 2009, and Truliant knee systems sold since 2017.
What is the Problem with Exactech Optetrak Knee Implants?
Like Exactech hip replacements, the problem with recalled Exactech knee implants is the polyethylene insert. Polyethylene is a form of plastic that is supposed to act in the same way cartilage does in your knee joint. When the insert fails, the knee replacement fails, causing pain, accelerated wear on the joint, bone loss, and fracture or fatigue of the replacement. In addition, revision surgery is needed, causing even more unnecessary pain, loss in wages, and loss of quality of life.
How Do I Know if My Knee or Hip Replacement is Failing?
Millions of people have joint replacements. There are several clues that indicate failure of these implants. Patients may experience:
- New or worsening pain
- Swelling
- Pain while walking
- Instability
- Noises like grinding or clicking
If you experience these issues, notify your physician. He or she will likely obtain x-rays or other testing to evaluate the situation.
How Do I Find Out if My Knee or Hip Replacement is an Exactech?
Medical records like this can be confusing, and most people don’t have records of the brand or type of replacement they have received.
If you have not already been notified about the recall from a physician, call or email your surgeon to find out what kind of hip or knee replacement you have received and if it is involved in a recall. If it is, you are likely eligible for a lawsuit.
Contact OnderLaw today!
Call 314-408-6424
from anywhere in the United States!
OnderLaw hip and knee replacement lawyers can help.
Our consultations are free.
You don’t pay a dime unless we win your case.
It is not necessary that you know whether you have a recalled Extactech knee or hip implant. We can investigate for you. Call OnderLaw today for a free, no-obligation consultation. If you are eligible, we can discuss moving forward with your claim.








 What is Behind Exactech Lawsuits?
What is Behind Exactech Lawsuits?